Long-Term Follow-Up of Left Atrial Appendage Exclusion: Results of the V-CLIP Multi-Center Post-Market Study
Zias, E. et al. (2025). Journal of Clinical Medicine. doi:10.3390/jcm14155473
Introduction
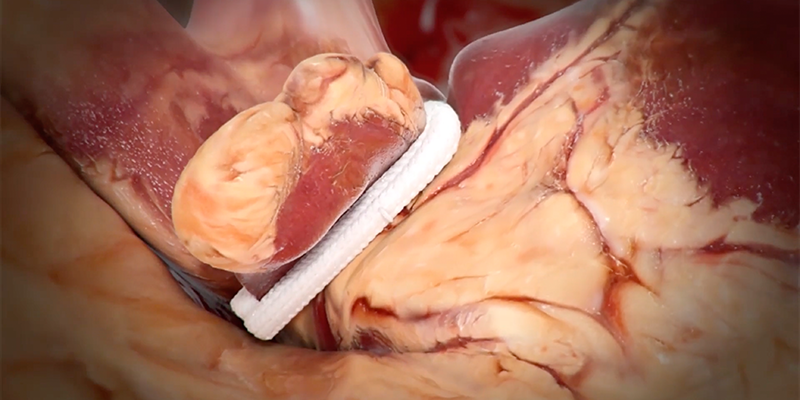
The V-CLIP multi-center post-market study led by Dr. Elias Zias and colleagues (New York University Langone Medical Center) evaluated long-term LAAE performance and safety of the epicardial V-shape AtriClip device.
Methods
This was a retrospective-prospective, multi-center post-market study (Clinicaltrials.gov: NCT05101993). Patients who received a V-shape AtriClip® device (AOD2 or V-Clip, AtriCure, Inc., Mason, Ohio) during non-emergent cardiac surgery and who consented to LAA imaging were followed for 12 months. Primary performance was LAAE without residual left atrium-LAA communication, assessed by computed tomography angiography or transesophageal echocardiography imaging at last follow-up. Primary safety was the incidence of device- or implant procedure-related serious adverse events (SAEs; death, major bleeding, surgical site infection, pericardial effusion requiring intervention, and myocardial infarction) within 30-days.
Results
A total of 155 patients underwent surgical LAAE with the AtriClip device (intent-to-treat [ITT] group), 151 of whom had evaluable imaging at 12-months (modified intent-to-treat [mITT] group). In the ITT group, mean age was 65.8 years, median CHA2DS2-VASc was three, and 78% were male. The most common cardiac surgical approach was sternotomy (57%), and the most common concomitant cardiac procedure was coronary artery bypass grafting (36%).
Primary safety was achieved, with 100% (155/155; 95% CI: 97.75%, 100%; p<0.0001) of patients free from a predefined set of device- or procedure-related SAEs within 30 days. Among 151 patients with evaluable imaging (mITT), primary performance was achieved with 100% (151/151) of patients with complete LAAE (95%CI: 97.59%, 100%; p<0.0001). A sub-analysis of primary performance endpoints by cardiac surgical procedure demonstrated complete LAAE in 100% of patients that underwent open sternotomy and minimally invasive surgery (right and left access).
No thrombi were observed in patients with residual stumps >10mm. Five (3.2%; 5/155) device- or procedure related SAEs occurred through 12 months (one device-related, four related to general cardiac procedures).
Limitations
- This was a non-randomized, single-arm study, which may have introduced selection bias and limited direct comparisons for safety and effectiveness.
- Women and racial/ethnic minorities are underrepresented in the enrolled patient population.
- The study design was neither designed or powered to assess a potential association between residual communication or LAA neck and thromboembolic events.
Key Takeaways
- The V-Clip AtriClip device demonstrated safe and successful long-term LAAE, supporting its use as a standard adjunct during cardiac surgery.
- Rates of LAAE without residual communication (leaks) were substantially higher than those reported for other surgical LAA closure techniques and endocardial occlusion devices, aligning with previously published data on the AtriClip.
